Product Overview
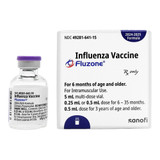
Fluzone® Trivalent 2025-2026 Influenza Virus Vaccine is an inactivated vaccine designed to protect against the seasonal influenza virus. Each 0.5 mL dose contains 45 mcg of hemagglutinin antigen from three different influenza strains, providing protection against the most common circulating strains for the 2024-2025 flu season. This vaccine is packaged in a 10-dose multidose vial, making it ideal for healthcare settings that need to vaccinate multiple individuals. Fluzone® Trivalent is recommended for individuals 6 months and older and is an essential tool in flu prevention efforts.
Product Features
-
Provides protection against the seasonal influenza virus, covering three strains for 2024-2025
-
Contains 45 mcg of hemagglutinin antigen per 0.5 mL dose for each of the three strains
-
Comes in a 10-dose multidose vial, ideal for healthcare settings
-
Suitable for individuals 6 months and older for flu prevention
-
Inactivated vaccine formulation for a safe and effective immune response
Product Specifications
| Catalog Number/SKU | MF-49281064115 |
| Application | Flu Vaccine |
| Container Type | Multiple-Dose Vial |
| Dosage Form | Injection |
| Generic Drug Name | Influenza Virus Vaccine, Trivalent Inactivated |
| NDC Number | 49281-0643-15 |
| Storage Requirements | Requires Refrigeration |
| Strength | 45 mcg / 0.5 mL |
| Type | Intramuscular |
| UNSPSC Code | 51201608 |
| User | Indicated for People 6 Months of Age and Older |
| Volume | 5 ml |